SAFETY
& DOSING
- In this section:
- Safety Profile
- Dosing
In adults with IBS-D
XIFAXAN has a well-established safety profile1
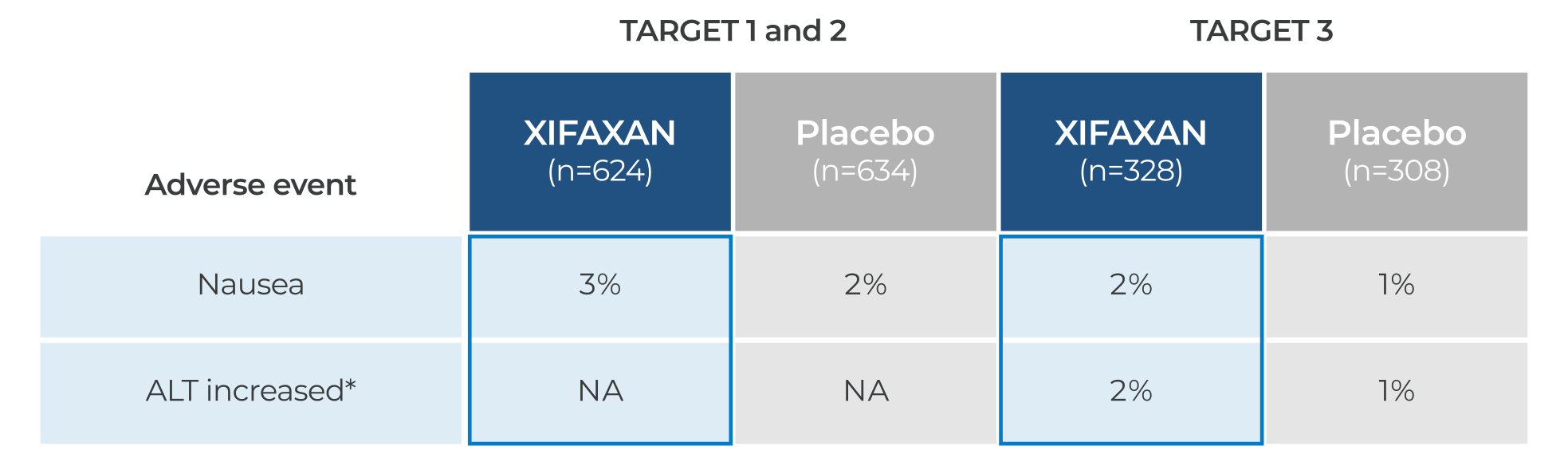
Side effects at rates similar to placebo

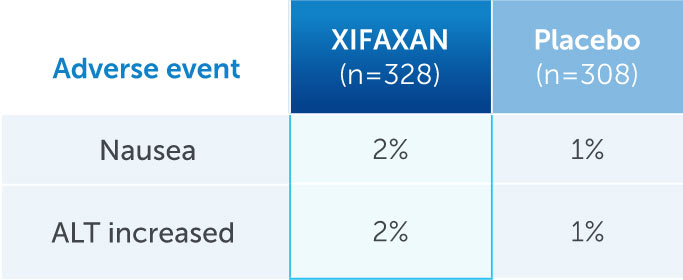

ALT, alanine aminotransferase; NA, not available.
*Most of the events of ALT increase were due to transient increases that resolved over time and were not temporally associated with study drug treatment.2
- Constipation was observed in 0.3%-0.6% of patients treated with XIFAXAN2,3
- Did not cause any clinically relevant antibiotic resistance after 1 to 3 treatment cycles4
- Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including XIFAXAN, and may range in severity from mild diarrhea to fatal colitis. If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued1
Dosing XIFAXAN: 2 weeks of treatment delivered
lasting relief of multiple symptoms1,2,5
Most treatments manage symptoms with continuous daily therapy,
but XIFAXAN is different1,6,7
One 550-mg tablet 3 times a day with or without food1
2 weeks of treatment, not continuous, daily prescription medication1
Patients who complete initial treatment can be retreated up to 2 times for recurrence1
Provided relief of multiple IBS-D symptoms: abdominal pain, diarrhea, bloating, and urgency1,5,8,9

XIFAXAN Dosing Flashcard
A summary of dosing information for prescribing XIFAXAN.
ICD-10 code for IBS-D10,†
K58.0 Irritable bowel syndrome with diarrhea
XIFAXAN 550 mg
3 times a day for 14 days
#42 tabletsCan retreat up to 2 times

XIFAXAN Dosing Flashcard
A summary of dosing information for prescribing XIFAXAN.
IBS-D, irritable bowel syndrome with diarrhea.
†The ICD-10 code and all other patient-access–related information are provided for informational purposes only. It is the treating physician’s responsibility to determine the proper diagnosis, treatment, and applicable ICD-10 code. Salix Pharmaceuticals does not guarantee coverage or reimbursement for the product.
References: 1. XIFAXAN. Prescribing information. Salix Pharmaceuticals; 2023. Accessed November 27, 2024. https://shared.salix.com/globalassets/pi/xifaxan550-pi.pdf 2. Lembo A, Pimentel M, Rao SS, et al. Repeat treatment with rifaximin is safe and effective in patients with diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2016;151(6):1113-1121. doi:10.1053/j.gastro.2016.08.003 3. Schoenfeld P, Pimentel M, Chang L, et al. Safety and tolerability of rifaximin for the treatment of irritable bowel syndrome without constipation: a pooled analysis of randomised, double-blind, placebo-controlled trials. Aliment Pharmacol Ther. 2014;39(10):1161-1168. doi:10.1111/apt.12735 4. Pimentel M, Cash BD, Lembo A, Wolf RA, Israel RJ, Schoenfeld P. Repeat rifaximin for irritable bowel syndrome: no clinically significant changes in stool microbial antibiotic sensitivity. Dig Dis Sci. 2017;62(9):2455-2463. doi:10.1007/s10620-017-4598-7 5. Pimentel M, Lembo A, Chey WD, et al; TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364(1):22-32. doi:10.1056/NEJMoa1004409 6. Viberzi. Prescribing information. Allergan; 2020. Accessed January 4, 2024. https://www.rxabbvie.com/pdf/viberzi_pi.pdf 7. Amitriptyline HCl. Prescribing information. Sandoz; 2020. Accessed November 27, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf 8. Schoenfeld PS, Brenner DM, Pichetshote N, Heimanson Z, Lacy BE. Rifaximin significantly improves bowel movement urgency in patients with irritable bowel syndrome with diarrhea: a pooled analysis of three phase 3 trials. Poster presented at: Digestive Disease Week; May 21-23, 2021; virtual. 9. Pimentel M, Cash BD, Lacy BE, Heimanson Z, Lembo A. Assessing the efficacy of rifaximin in diarrhea-predominant irritable bowel syndrome: a post hoc analysis of two phase 3, randomized, placebo-controlled trials. Poster presented at: World Congress of Gastroenterology; October 13-18, 2017; Orlando, FL. 10. ICD-10. Centers for Medicare & Medicaid Services. Updated September 26, 2024. Accessed November 27, 2024. www.cms.gov/Medicare/Coding/ICD10
INDICATIONS
XIFAXAN® (rifaximin) 550 mg tablets are indicated for the reduction in risk of overt hepatic encephalopathy (HE) recurrence in adults and for the treatment of irritable bowel syndrome with diarrhea (IBS-D) in adults.
XIFAXAN® (rifaximin) 550 mg tablets are indicated for the treatment of irritable bowel syndrome with diarrhea (IBS-D) in adults.
IMPORTANT SAFETY INFORMATION
- XIFAXAN is contraindicated in patients with a hypersensitivity to rifaximin, rifamycin antimicrobial agents, or any of the components in XIFAXAN. Hypersensitivity reactions have included exfoliative dermatitis, angioneurotic edema, and anaphylaxis.
- Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including XIFAXAN, and may range in severity from mild diarrhea to fatal colitis. If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued.
XIFI.0125.USA.23V3.0